Pediatric Pathology
PHOX2B (EP312)
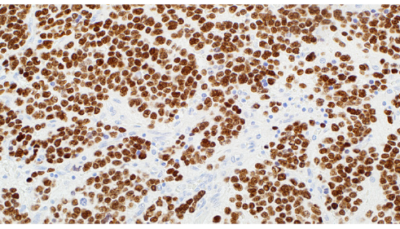
Paired-like homeobox 2B (PHOX2B) is a transcription factor located
on chromosome 4p13 which is crucial to the formation of autonomic
ganglia in the autonomic nervous system (ANS). PHOX2B gene
is strictly expressed in neural crest derivatives committed to the
noradrenergic phenotype. The PHOX2B gene encodes a paired-like
homeo-domain transcription factor with an extra-axial expression
pattern restricted to the ANS. Neuroblasts of peripheral neuroblastic
tumors are derived from the sympathoadrenal lineage, a division
of the ANS. PHOX2B has been observed in peripheral neuroblastic
tumors, neuroblastomas, paragangliomas, ganglioneuroblastomas,
ganglioneuromas and pheochromocytomas. PHOX2B has been
reported to be negative in other small round blue cell tumors.
CITED1 (5H6)
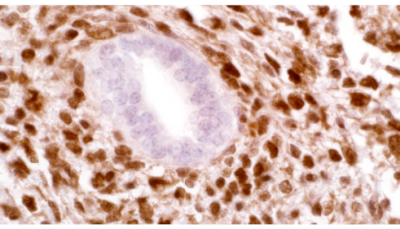
CITED1 is a transcriptional cofactor expressed in the metanephric
mesenchyme (MM) of the embryonic kidney and is downregulated
as these cells undergo epithelial differentiation. It is
thought that CITED1 may play a role in maintaining MM cells in an
undifferentiated state. Wilms’ tumors (WT) are thought to arise
from abnormal postnatal retention and dysregulated differentiation
of nephrogenic progenitor cells that originate as a condensed MM
within embryonic kidneys. CITED1 expression has been shown to
persist in blastemal cell populations of human WT. In the developing
embryonic kidney, CITED1 expression is seen in the cytoplasmic
compartment. In WT, expression of CITED1 is detected in the
nuclear compartment of tumor cells. It has been suggested that
persistent expression of CITED1 in the MM could play a role in WT
initiation and pathogenesis. CITED1 has been detected in 86.8% of
WT Cases.
NKX2.2 (EP336)
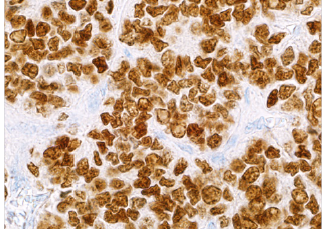
NKX2.2 is a homeodomain transcription factor that plays a role in neuroendocrine and glial differentiation. NKX2.2 is upregulated in Ewing’s sarcoma as a result of the oncogenic EWS-FLI1 fusion protein. As one of many small round blue cell tumors, Ewing’s sarcoma can be difficult to diagnose due to the characteristic undifferentiated morphology. NKX2.2 has proven its utility as a sensitive maker for distinguishing Ewing’s sarcoma from other round blue cell tumors when used in a panel.
CD99 (EPR3097Y)
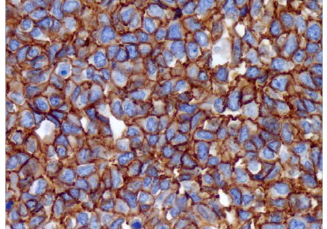
CD99, as detected with a variety of antibodies, is expressed by virtually almost all Ewings sarcoma and primitive peripheral neuroectodermal tumors (ES/PNET) and demonstrates strong and diffuse membranous staining. Other tumors that may show CD99 expression include neuroendocrine carcinomas, mesenchymal chondrosarcomas, solitary fibrous tumors, synovial sarcomas, vascular tumors, small round blue cell tumors, lymphoblastic lymphoma, acute myeloid leukemia, and myeloid sarcoma. However, strong and diffuse membranous reactivity for CD99 favors ES/PNET over the other diagnostic considerations. The other CD99+ tumors usually show cytoplasmic and more heterogeneous staining. Therefore, when making a final diagnostic interpretation, CD99 must be considered in a panel with other antibodies.
TFE3 (MRQ-37)
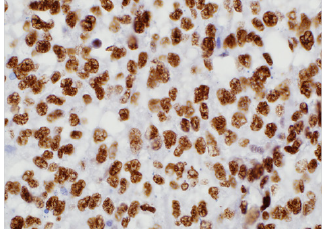
Transcription factor E3 (TFE3) is a protein expressed in many cell types that are encoded by the TFE3 gene. This gene may be involved in chromosomal translocations that occur in some cancers. Xp11 translocation renal cell carcinomas (RCC) are a recently recognized subset of RCC, characterized by chromosome translocations involving the Xp11.2 break point and resulting in gene fusions involving the TFE3 transcription factor gene that maps to this locus. Alveolar soft part sarcoma (ASPS) is an uncommon soft tissue sarcoma of uncertain differentiation. The hallmark of ASPS is a chromosomal rearrangement at 17q25 and Xp11.2 engendering an ASPSCR1-TFE3 fusion gene responsible for an aberrant transcription factor presumably enabling pathogenesis.
INI-1 (MRQ-27)
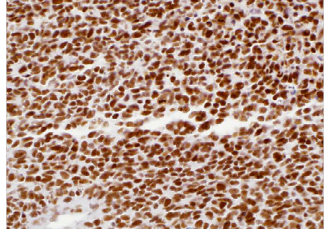
The INI-1 gene, which encodes a functionally uncharacterized protein component of the hSWI/SNF chromatin remodeling complex, is often mutated or deleted in malignant rhabdoid tumor (MRT). Two isoforms of INI-1 that differ by the variable inclusion of amino acids are potentially produced by differential RNA splicing. The morphology of MRTs can present challenges in differential diagnosis. The overall survival of MRTs relative to its potential mimics [medulloblastoma, supratentorial primitive neuroectodermal tumors (sPNETs)] is quite low, and thus differentiation from these other tumors is desirable. Lack of nuclear labeling by anti-INI-1 is characteristic of MRT. The majority of medulloblastomas and sPNETs are labeled by anti-INI-1. MRTs also originate from the kidney and soft tissues.
