On August 29, 2024, Johnson & Johnson announced the submission of the first FDA application for nipocalimab, an investigational treatment targeting myasthenia gravis (MG).
Learn more in the full article here.
Myasthenia gravis (MG) is a neuromuscular disorder that causes muscle weakness, affecting the eyes, eyelids, facial expressions, chewing, swallowing, speaking, and other muscles.
Nipocalimab stands out as the first and only FcRn blocker designed to reduce autoantibodies, delivering sustained disease control. This was demonstrated through improvements in Activities of Daily Living for MG patients participating in the phase 3 Vivacity-MG3 study.
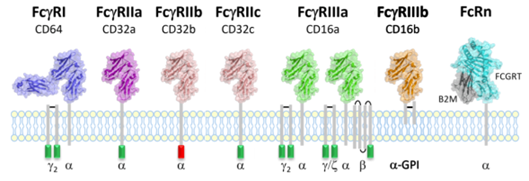
FcRn-targeted therapies for autoimmune diseases offer great potential. We are thrilled to provide a comprehensive range of human Fc gamma receptors and FcRn products to support your drug development research.
The products from our supplier the Fc receptor specialist Gamma Proteins are of extremely high purity and with full biological activity confirmed by surface plasmon resonance (SPR). In addition of the unconjugated proteins, recombinant Fc gamma receptors and FcRn are also available in biotinylated proteins.