Therapeutic Drug Monitoring ELISA kits
Krishgen currently manufactures 350+ ELISA, with targets covered for quantitative detection in fields of oncology, auto-immunity, diabetes and several more. The largest range in the world.
The KRIBIOLISA range of Therapeutic Drug Monitoring ELISA kits can be used for efficient biological drug testing by accurately monitoring serum trough levels and the presence of anti-drug antibodies respectively. The kits are also CE IVD marked for diagnostic use.
What sets Krishgen TDM and ADA ELISA apart:
- Krishgen TDM kits are calibrated against the innovator / commercial drug itself
- When possible, they are calibrated against international NIBSC standards from WHO.
- Each product is validated as per FDA guidelines for bioassays.
- The use of specific idiotypic antibodies that ensure a higher degree of specificity and sensitivity
- Validated for both serum and plasma, with spiking data available in our datasheets
- Ready To Use with lyophilized standards
- Precision CV < 15>
- Robust, with high lot-to-lot reproducibility
- 96 well plates, break apart strips includes calibrator set ready to use component
What are Anti-Idiotypic antibodies?
Krishgen uses anti idiotypic antibodies in TDM assays to ensure a very high degree of specificity. When one antibody binds to an idiotope of another antibody it is referred to as an anti idiotypic antibody. The variable part of an antibody including the unique antigen binding site is known as the idiotype. The combination of epitopes within the idiotype (i.e. the idiotopes) is unique for each antibody, see figure 1.
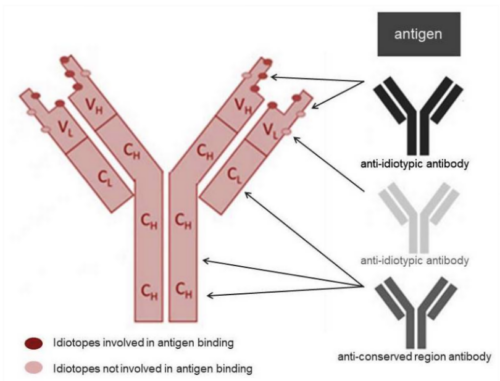
Fig 1. Antibody idiotope - the unique set of antigenic determinants of the variable region of an antibody. Because most therapeutic monoclonal antibodies developed today are human or humanized, the most likely immunogenic epitopes for the induction of anti-drug antibodies (ADA) lie within the hypervariable complementarity determining regions (CDR) that provide the majority of the binding contacts. Anti-idiotypic antibodies can be generated to bind specifically to one monoclonal antibody drug. These highly specialized antibodies can be used to set up pharmacokinetic (PK) assays in different formats to measure free or total drug levels in preclinical and clinical samples, or as positive controls in ADA assays.



