(By Keith Williams, Ph.D. - Business Manager, Analytical Standards Europe, Cayman Chemical)
Which format should I choose for a reference material?
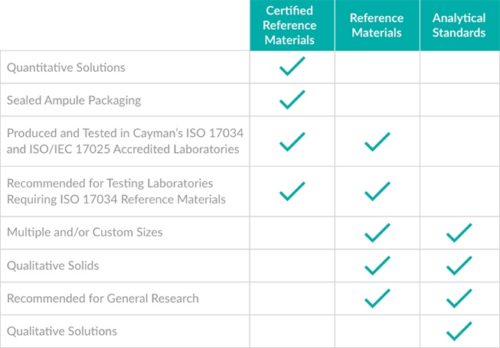
The choice of a solid versus solution reference material is driven by technical requirements, your quality system, perceived economics, and personal preferences. There is no single right form of a reference material that will meet everyone’s needs.
Some analytical techniques need a reasonable quantity of a solid product to enable an effective analysis to be carried out. For example, only a few grains of solid material are required for ATR-FTIR analysis. There is no benefit to having the material in a pre-made solution. Cayman’s analytical standards or reference material products best fit this need.
If you are after convenience and efficiency, you can choose one of the ready-made solutions. These are particularly advantageous if you are planning to use the product to spike into matrix or inject into a chromatographic system. The concentration provided on the Certificate of Analysis (CofA) of the Certified Reference Materials (CRMs) accurately and precisely reflects the true value of the concentration, along with its associated uncertainty. The solution CRMs also take into account purity and any salt form (if present). The solution can then be simply dispensed into new containers and diluted appropriately. This mechanism of preparing a working standard takes far less time than weighing out a solid material, releasing your valuable time for other activities.
To save even more time in preparing working standards, you could consider using one of the pre-made mixtures. Crack the ampoule, dilute as needed, and inject it into your instruments, potentially saving hours of your time. And if you happen to need something not listed in the catalogue, Cayman Chemical might be able to make a custom mixture for you.