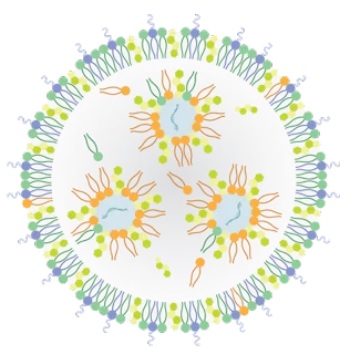
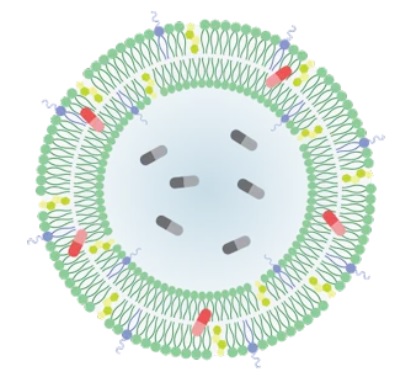
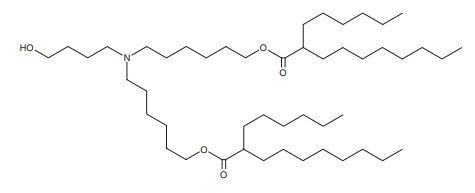
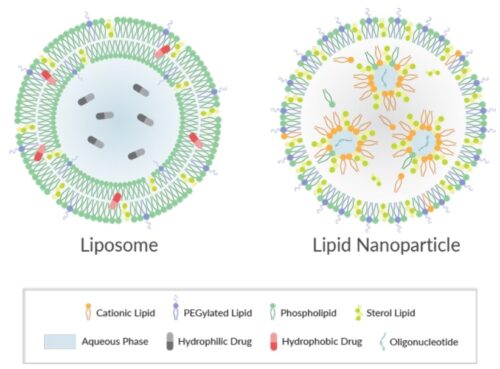
About Lipid-Based Drug Delivery (LBDD)
Lipid-based formulations have contributed to significant achievements in drug development and the delivery of therapeutic biomolecules and genes. Their design can help overcome the biodistribution and/or bioavailability limitations of certain conventional drug delivery methods by enabling cell-specific targeting and transport to specific organelles and prolonging the stability of the drug. Lipid-based nanoparticles can be used to package drugs for topical, oral, intravenous, or pulmonary routes of administration. The first FDA-approved, lipid-based drug formulation, Doxil®, an antitumor antibiotic, became available in 1995. Since then, seventeen additional FDA-approved therapeutics are on the market for use in cancer, gene therapy, antiviral vaccines, fungal diseases, analgesia, and photodynamic therapy. Hundreds more are in clinical trials or even authorized for emergency use—as is the case for the Pfizer and Moderna COVID-19 vaccines. Indeed, the most progress has been made in the field of nucleic acid delivery, involving the encapsulation of short interfering RNA (siRNA), microRNA (miRNA), short activating RNA (saRNA), or messenger RNA (mRNA) in a lipid nanoparticle for gene silencing or activation and protein production.