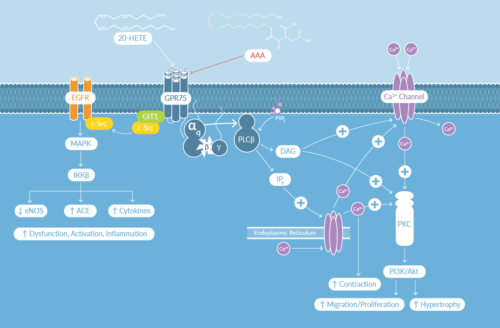
G protein-coupled receptor 75 (GPR75) has been identified as a 20-HETE (20-Hydroxyeicosatetraenoic acid) receptor. It signals through Gq/phospholipase C (PLC)/protein kinase C (PKC) and c-Src/EGFR pathways (Figure 1). Previously GPR75 was deorphanized as an inflammatory chemokine receptor when CCL5/RANTES was identified as its ligand. Through GPR75, RANTES was shown to activate MAPK signaling to protect hippocampal HT22 cells from amyloid-β-induced cell death and to stimulate insulin secretion in pancreatic islet cells.1,2 Now it has been established that 20-HETE, a member of the cytochrome (CYP) P450-derived eicosanoids, acts through the same receptor to elicit vascular effects.3
-
Research area
- Biochemicals
- Blood and Biospecimens
- Cell biology
- Environmental
- Flow Cytometry
- Forensic Science
- Genomics
- Immunology
- Labware
- Microbiology
- Pathology
- Transplantation
429 Too Many Requests 429 Too Many Requests
nginx - Products
- Suppliers
- About us
- Resources
- Events
- Support
- Lab Services
- Promotions