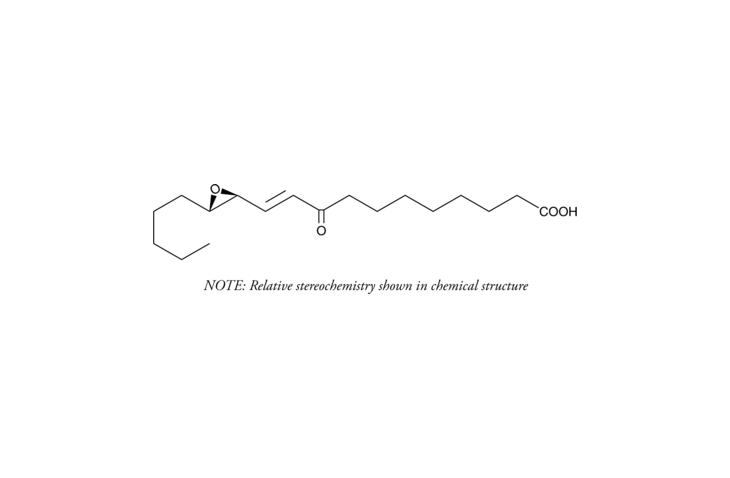
During oxidative stress, the abundant unsaturated fatty acid linoleic acid undergoes lipid peroxidation to produce ?,?-unsaturated epoxy-keto-octadecenoic acids (EKODEs). Nonenzymatic autooxidation of linoleic acid generates six major EKODE isomers, which differ from one another in the positioning and the orientation of the epoxy group relative to the keto moiety. trans-EKODE-(E)-Ib is a biologically active peroxidation product of linoleic acid that is characterized, structurally, by having a trans carbon-carbon double bond between the 9-keto and 12,13-epoxy groups. It activates an antioxidant response element (ARE) in neuronal cells and induces the expression of ARE-regulated cytoprotective genes like NQO1.{17702} This EKODE also stimulates the synthesis of aldosterone and corticosterone in adrenal cells when supplied at 1-5 ?M.{17699,17700} This effect appears to be mediated by a rise in intracellular calcium.{17701}
Do you have any questions about this product?
Order your product by email
Productname
trans-EKODE-(E)-Ib
10004224-500
By filling out this form, you are placing an order by e-mail. You will receive an order confirmation within one working day. The order cannot be modified after receipt of the order confirmation.
Request a sample
Productname
trans-EKODE-(E)-Ib
10004224-500
By filling out this form, you request a sample. You will receive an order confirmation within one working day. The order cannot be modified after receipt of the order confirmation.
Are you looking for specific products, alternatives or documentation?